3 Nuclear Magnetic Resonance
A charge particle in movement generates a magnetic field, as electrons, nuclei generates a magnetic moment due to his spin. When the nuclear magnetic moment is placed in an external magnetic field, his spin state suffer a transition, different spin states gives different magnetic potential energy. In the presence of the static magnetic field which produces a small amount of spin polarization, a radio frequency signal of the proper frequency can induce transition between spin states. This "spin flip" places some of the spins in their higher energy states. If the radio frequency signal is then switched off, the relaxation of the spins, back to the lowe energy state, produces a measurable amount of RF signal at the at the resonant frequency associated with the spin flip. This process is called nuclear magentic resonance NMR.
|
|
3.1 Notes to calculate a NMR.
Due to fact that NMR involves the nuclei, we must have a special threatment in the calculation. Here are some points we have to care to calculate NMR chemical shifts (CS).
It is recomended to calculate the NMR CS with several XC functionals that were specially done to study this phenomena. (RPBE, PBE0, BP86)
Also, the Basis Set has to be choosen carefully. ORCA manual recomends to use EPR and IGLO basis sets, which were done specifically for NMR calculations.
To compare NMR experimental data, first you have to calculate NMR shielding of the reference molecule that was used on the experiment. For example a very popular reference molecule for H NMR CS is the Tetramethylsilane (TMS) .
|
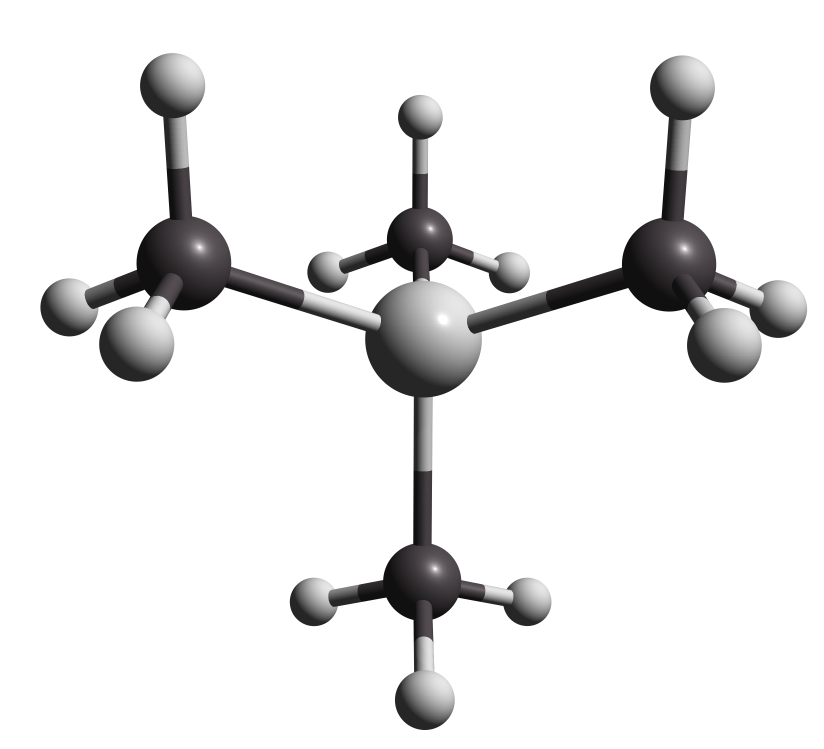
Figure 3.1. TMS reference molecule for H NMR CS
|
3.2 Lets calculate H NMR CS
Build the structrure of the CH4 and HCN molecules.
To acquire the NMR CS we have to insert the next lines:
%eprnmr ori IGLO #alternative OwnNuc
LocMet FB #localization method for IGLO
#FB=Foster-Boys PM=Pipek-Mezey
Nuclei = all { shift }
end
In this excersise we are going to use the PBE0 XC functional with the IGLO-II basis set.
Inputs should be like these: ch4.inp hcn.inp |
Figure 3.2.1 Methane (CH4)
Figure 3.2.3 Formon Nitrile (HCN)
|
3.3 NMR CS output
To locate the isotropic chemical shielding on the output file, we have to search for the /CHEMICAL SHIFT string line.
There you will find the orientation and egienvalues of the tensor. The total isotropic shielding is reported as shown in figure 3.3.1.
|

Figure 3.3.1. Outputo of the NMR analysis |
3.4 Comparison with experimental data
The are very little experimental data about absolute nuclear shielding, the gross of this data are comparison as told before. Here we present a little table of absolute shieldings and chemical shifts differences for H NMR CS. The molecule of reference is TMS.
| ATOM |
AS exp |
AS theo |
CS exp |
CS theo |
| TMS |
31.1 |
31.9 |
0.00 |
0.0 |
| CH4 |
30.6 |
31.9 |
0.22 |
0.0 |
| HCN |
27.8 |
29.8 |
3.78 |
2.1 |
AS- Absolute Nuclear Shielding
CS- Chemical Shift (TMS as reference)
|
|